

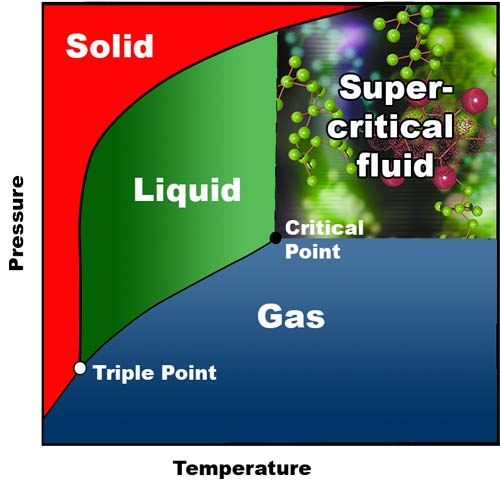
 A Supercritical fluid is one where, at certain temperature and pressure conditions, liquids and gasses reach exactly the same density and so mix together completely forming a new state that combines both liquid and gaseous properties.
A Supercritical fluid is one where, at certain temperature and pressure conditions, liquids and gasses reach exactly the same density and so mix together completely forming a new state that combines both liquid and gaseous properties.
Supercritical CO2 is a "Green" solvent used for processing ultra-pure pharmaceutical and food products. Supercritical CO2 allows you to refine compounds without either leaving toxic residues behind or risking thermal degradation of the products.
Traditional organic solvents have been used for many decades but carry with them high costs plus significant health, environment and safety hazards.
Supercritical CO2 is now used in a large number of applications - from the processing of coffee and vegetable oil through to producing insulin and anti-asthma drugs. It can be used for large-scale power generation as well as for low porosity oil-bearing rock cores and hydrocarbon extraction, and even to produce Biocrude, a next-generation non-fossil fuel. Additionally, modern food and pharmaceutical powder generation processes often involve crystallisation using CO2 in its supercritical state.
The need for new and clean technologies for processing ultra-pure pharmaceutical and food products has led to a rapid rise in the use of CO2 - which allows users to refine compounds without either leaving toxic residues behind or risking thermal degradation of the products. The alternative of traditional solvents carries with it high costs together with significant health, environment and safety hazards.
However, the vapour permeability of both the processes' materials and the materials being refined, become increasingly important and we, at Versaperm, have developed an instrument and technique to measure this both quickly and accurately.
The system is fast, accurate and easy to use.
The advantage of supercritical CO2 is that it can permeate deep into a material's matrix and extract the desired compound more efficiently than by using conventional organic solvents.
 Food applications
Food applications
Supercritical fluids have liquid-like solvent power and gas-like diffusivity. Supercritical carbon dioxide is used by the food industry because of its lack of toxicity, lack of flammability, low cost, wide availability, its solvent recoverability, plus the ease of achieving a supercritical temperature (31.1°C) and pressure (7.4MPa).
Supercritical fluid extraction (SFE) overcomes the limitations of solvent residues and restrictions on the use of conventional solvents in food processing. Additionally, particulate products can also be produced by means of supercritical fluid (SCF) processing. These processes are non-flammable, non-toxic, non-polluting and offer recoverable characteristics
Applications of supercritical fluid extraction already go well beyond simply producing coffee or extracting high purity compounds from barbados nuts, berries, black currant, blueberries, brazilian propolis, canola, chamomile, cocoa, coriander, cranberry, crowberry, dodder, elderberry, elderberry, emburana, five-flavour berry, grape, hop, macela, marigold, maypop, olive, onion, passionflower, pigeon pea, pine tree, raspberry, rice, rooibos, shiyacha, skullcap, sour cherry, soybean, spearmint, stonebreaker, sweet grass, tea, wild cherry and wormwood.
Pharmaceutical applications
Pharmaceutical companies use production processes, such as SFE, which offer a low environmental impact, reduce the use of volatile organic compounds and avoid residues in the finished product.
The principal pharmaceutical use of supercritical fluids involve
Three main processes are employed in the production of fine and monodisperse powders
LINKS
For a press release on Supercritical CO2 Click here
Wikipedia - Supercritical CO2
Supercritical fluid extraction: recent advances and applications