

 Measuring the permeability of drug measured release patches and packaging
.
Measuring the permeability of drug measured release patches and packaging
.
The key factor in slow release drug patches is permeability, which controls the rate of flow of the drug into the patient. Transdermal drug patches are usually made from a polymer material which holds back a chemical reservoir that is loaded beyond its solubility.
The drug transfers from a reservoir through a polymer membrane, enabling the drug to permeate through the skin when it is in contact. The chemical diffuses through both the membrane and the skin barriers and on into the body, with the rate of delivery depending on both the concentration gradient across the barriers and the permeability of each.
Due to a deliberate over solubility within the reservoir, the delivery of the drug usually follows a burst and lag mechanism with a high initial permeation or transfer rate as the patch is pressed onto the skin. However, as time moves on a stable concentration gradient is established and delivery of the drug settles to a reasonably regular and constant flow.
The permeability, which can be measured in the PPM range with our permeability measurement equipment, is crucial in drug delivery and is used in cases such as the Ocusert system.
Versaperm equipment can be used to measure this permeability for both drug release patches and medical gas sush as used in anaesthetic equipment.
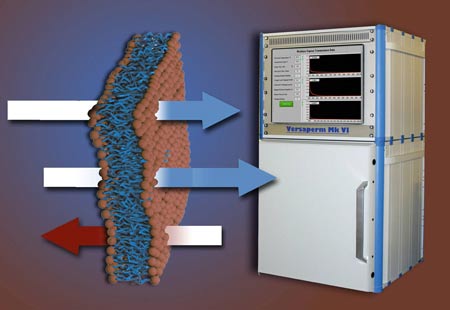 Medical packaging and permeability - from Unitary dose blister packs
Medical packaging and permeability - from Unitary dose blister packs
Water is the single most damaging contaminant that affects blister packaging as water vapour can permeate through the wall of the blister. The rate depends not just on the permeability of the blister material, but also on the manufacturing process. Blow moulding, for example, can increase the rate of contamination increase by a factor of four.
Both the loss or gain of water (or other contaminants) can have potentially significant effects to the products within blister packs. Water loss may lead to a change in the physical properties of a solid preparation, or a change in viscosity and concentration of vital components in liquid products. Water gain can create contamination and causesensitive ingredients to swell. Powders intended for inhalation are particularly sensitive as their particle size is critical for correct delivery - and is significantly influenced by the moisture content.
The Versaperm equipment can measure the permeability not just for water vapour but of almost and gas / vapour. What’s more it is suitable for both flat samples of the polymer (or foil) and the finished blister.
 Sterilised instruments and wound dressings
Sterilised instruments and wound dressings Our equipment can measure the vapour permeability of virtually any type of packaging - from a foil sachet to a wound dressing or from the packaging around a sterilised syringe through to pots, tubs and even the valves in asthma inhalers. It can be used for product development in a development or research lab through to a QC system on a factory flow.