Pervapouration – separation by selectively permeable membranes
Pervapouration is a membrane-based separation process used to separate mixtures of liquids by partial vapourization through a selectively permeable membrane.
How It Works: - Role of Vapour Permeability
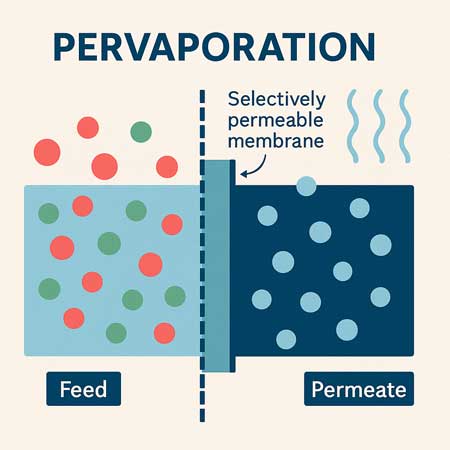
In pervapouration, vapour permeability is the key performance factor. The membrane’s ability to selectively allow one component to permeate in vapour form is what drives separation.
Two Key Factors:
- Permeability (P): How easily a molecule passes through the membrane.
- Selectivity (α): The membrane’s ability to prefer one component over another.
Mechanism of Transport (Solution-Diffusion Model)
- Sorption: The preferred component dissolves into the membrane at the feed side.
- Diffusion: It diffuses through the membrane due to the concentration gradient.
- Desorption: It evaporates on the low-pressure (permeate) side.
Solvent molecules that dissolve well and diffuse quickly have high vapour permeability in this context.
Membrane Materials Used
Depending on what you want to separate, the membrane material is tailored to favour certain types of solvents:
Membrane Material |
Best For Separating |
Permeation Preference |
PVA (Polyvinyl alcohol) |
Water from organics (dehydration) |
Hydrophilic |
PDMS (Polydimethylsiloxane) |
Organics from water (e.g., toluene, ethanol) |
Hydrophobic |
PEBA (Polyether block amide) |
Aroma recovery, alcohols |
Moderate polarity |
Zeolite or MOF mixed membranes |
Enhanced selectivity |
Customizable |
Industrial Applications of Pervapouration (Using Vapour Permeability)
 1. Dehydration of Alcohols
1. Dehydration of Alcohols
- Example: Ethanol–water mixtures
- Membrane: Hydrophilic (PVA)
- Why: Traditional distillation is inefficient near azeotropic points; pervapouration breaks the azeotrope.
2. Recovery of Volatile Organic Compounds (VOCs)
- Example: Toluene, acetone from wastewater
- Membrane: Hydrophobic (PDMS)
- Why: VOCs permeate faster due to higher solubility and diffusivity.
3. Flavour and Aroma Recovery
- Example: Orange juice or beer aroma recovery
- Membrane: Tailored for aroma compounds
- Why: Sensitive compounds can be recovered without high heat.
4. Fuel Processing
- Example: Removing water from bioethanol before blending with gasoline
- Membrane: Pervapouration membrane modules used inline with fuel production.
Performance Metrics
- Permeation Flux (J): Amount of substance passing through per unit area and time (e.g., g/m²·h).
- Separation Factor or Selectivity (α): Ratio of concentrations in permeate vs. feed.
- Permeability Coefficient (P): Combines diffusivity and solubility (measured in Barrer or GPU).
Engineers balance high permeability (good throughput) and high selectivity (effective separation).
Challenges & Optimization
- Trade-off between permeability and selectivity: Higher permeability sometimes reduces selectivity.
- Membrane swelling: Solvent absorption can degrade mechanical integrity.
- Membrane fouling: Especially in industrial feed streams with impurities.
- Thermal and chemical stability: Important for long-term operation.
Comparison to Other Techniques
Method< |
Energy Use |
Selectivity |
Scalability |
Distillation |
High |
Moderate |
High |
Pervapouration |
Lower |
High |
Moderate |
Adsorption |
Variable |
High |
Variable |
Reverse Osmosis |
Lower |
Poor for organics |
High |
Pervapouration membranes exploit vapour permeability as a controlled and selective process for separating volatile solvents or removing water from mixtures. Unlike other fields where vapour permeability is a leakage concern, here it is an engineered advantage.
International Standards Related to Pervaporation
 1. ISO (International Organization for Standardization)
1. ISO (International Organization for Standardization)
- ISO 16649-1 / ISO 16649-2 (and related)
While these are primarily microbiological standards, they influence membrane testing indirectly in bioprocessing where pervaporation may be used.
- ISO 15105-1 / ISO 15105-2
Plastics – Film and sheeting – Determination of gas transmission rate
These are crucial for vapour and gas permeability testing of barrier materials, including membranes used in pervaporation.
- ISO 2528
Sheet materials – Determination of water vapour transmission rate
A standard often adapted to compare or evaluate hydrophilic membranes used in pervaporation.
2. ASTM International
- ASTM E96 / E96M
Standard Test Methods for Water Vapour Transmission of Materials
Widely used in evaluating membrane materials before being used for pervaporation.
- ASTM D3985
Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting
Often cited in comparative studies involving pervaporation membranes.
- ASTM F1249
Standard Test Method for Water Vapour Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor
Relevant for evaluating membrane selectivity and barrier characteristics.
3. DIN Standards (Germany)
- DIN 53122-1
Water vapour permeability of plastic foils and coated textile fabrics
Useful for characterising membranes used in vapour phase separations.
4. JIS (Japanese Industrial Standards)
- JIS K7129 / K7130
Testing of gas permeability of films and sheets
Similar applications as ASTM D3985, often used for membranes in pervaporation and gas separation.
5. EN Standards (European Norms)
- EN 1939
Plastics – Film and sheeting – Determination of water vapour transmission rate using a dish method
Applied in contexts involving pervaporation where polymeric membranes are involved.
Other Considerations
- While there is no single ISO or ASTM standard dedicated exclusively to pervaporation as a method, many membrane and vapour permeability standards are regularly adapted in pervaporation research and industry.
- Pervaporation systems are often validated against these composite standards depending on the application (e.g. desalination, organic removal, alcohol dehydration, etc.)



 1. Dehydration of Alcohols
1. Dehydration of Alcohols 1. ISO (International Organization for Standardization)
1. ISO (International Organization for Standardization)